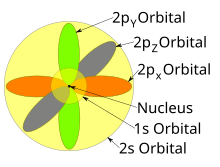
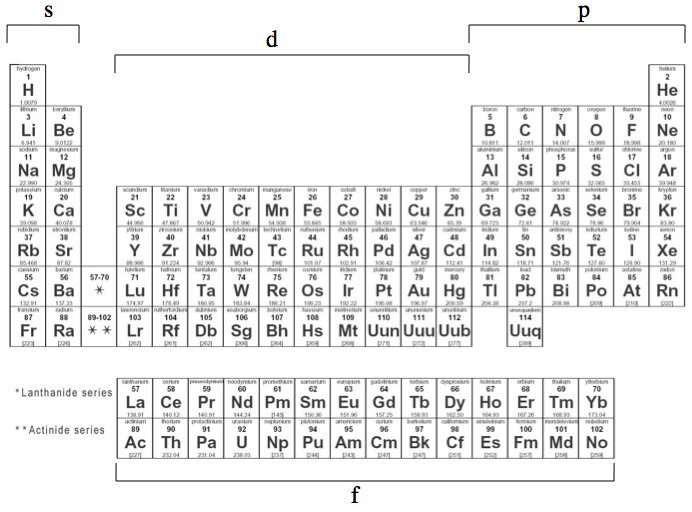
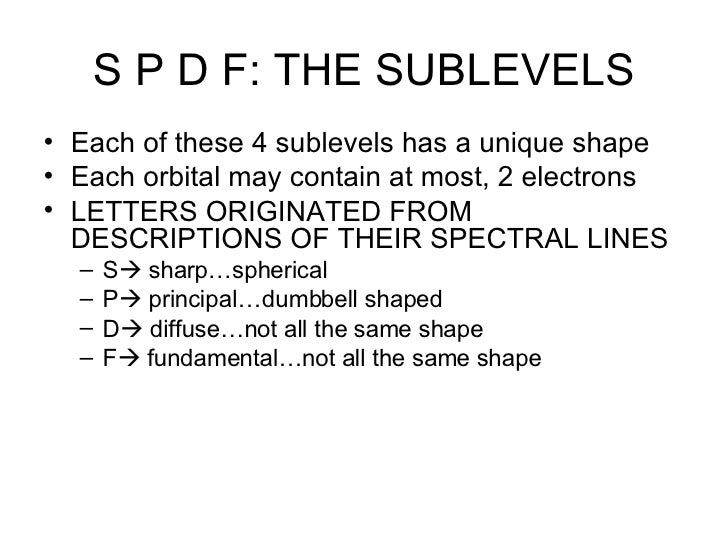
S p d f orbitals shapes names 236089-What are the shapes of s p d and f orbitals
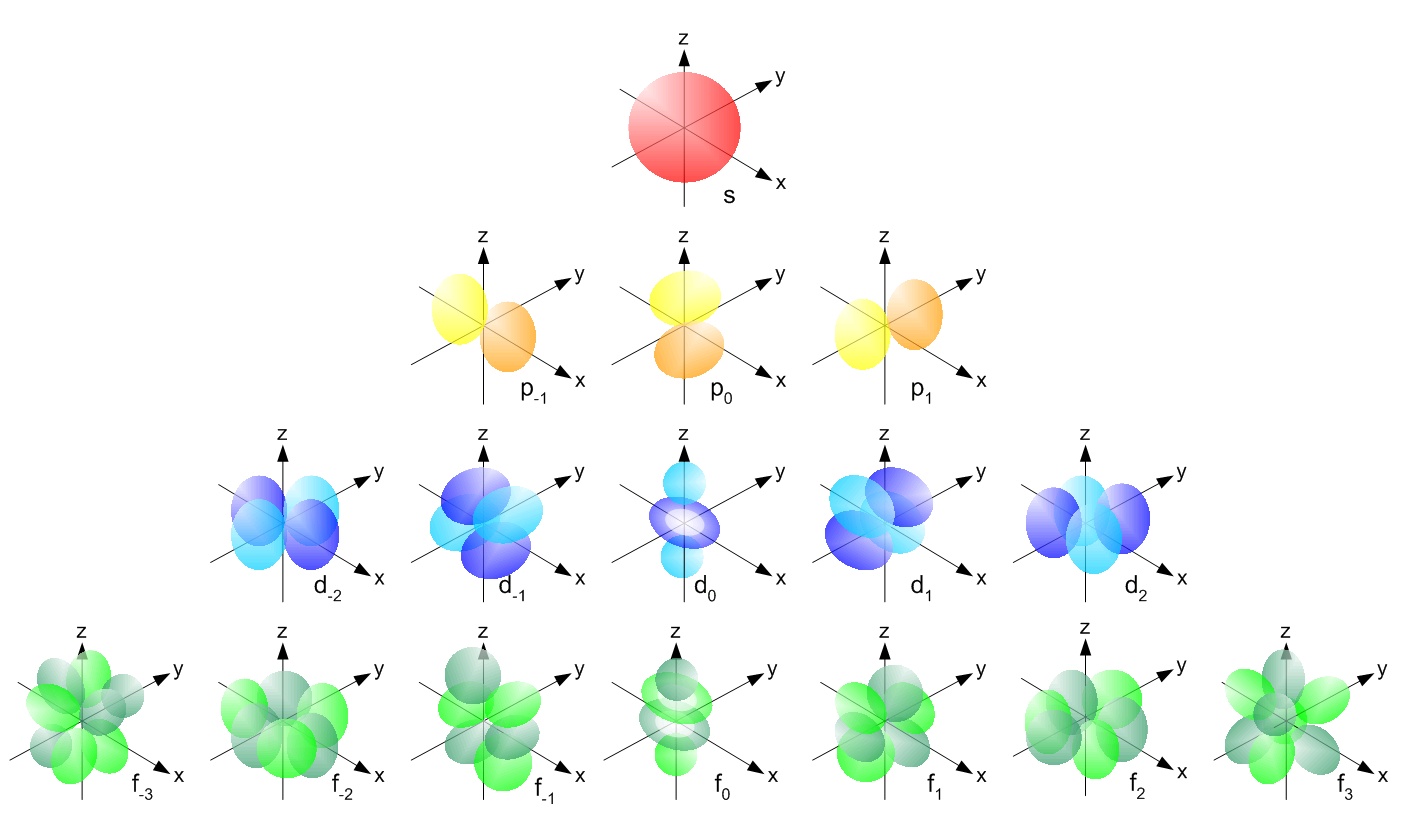
S orbital electrons will have a lesser amount of energy (more negative) than that of p orbital electrons which will have lesser energy than that of d orbital electrons As the extent of shielding from the nucleus is different for electrons in different orbitals, it leads to the splitting of energy levels having the same principal quantum number2dxy, 3dxz, 3dyz, 3d(xy)^2 and 3dz^2Are to arrange an orbital of that shape around the nucleus "s" subshell One possible orientation "p" subshell Three possible orientations There are five possible orbitals in a "d" subshell, and 7 possible orbitals in an "f" subshell!
Sublevel Spdf Chart Fgkb Katasekan Site
What are the shapes of s p d and f orbitals
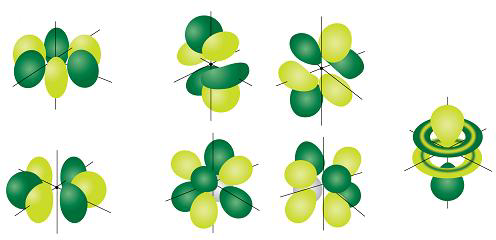
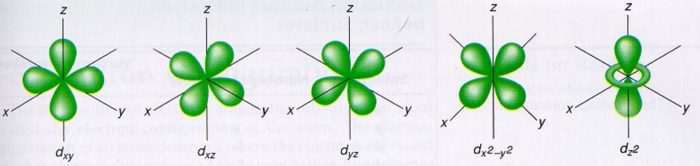
What are the shapes of s p d and f orbitals-For example, 3d xy, 3d yz, 3d zx, 3d x 2y 2The porbitals of higher energy levels have similar shapes although their size are bigger Shape of dorbitals For dsubshell, l = 2, there are five values of m namely 2, 1, 0, 1, 2 It means d orbitals can have five orientations These are represented by d xy, d yz, d zx, d x 2y 2 and d z 2;


S P D F Orbitals Chemistry Socratic
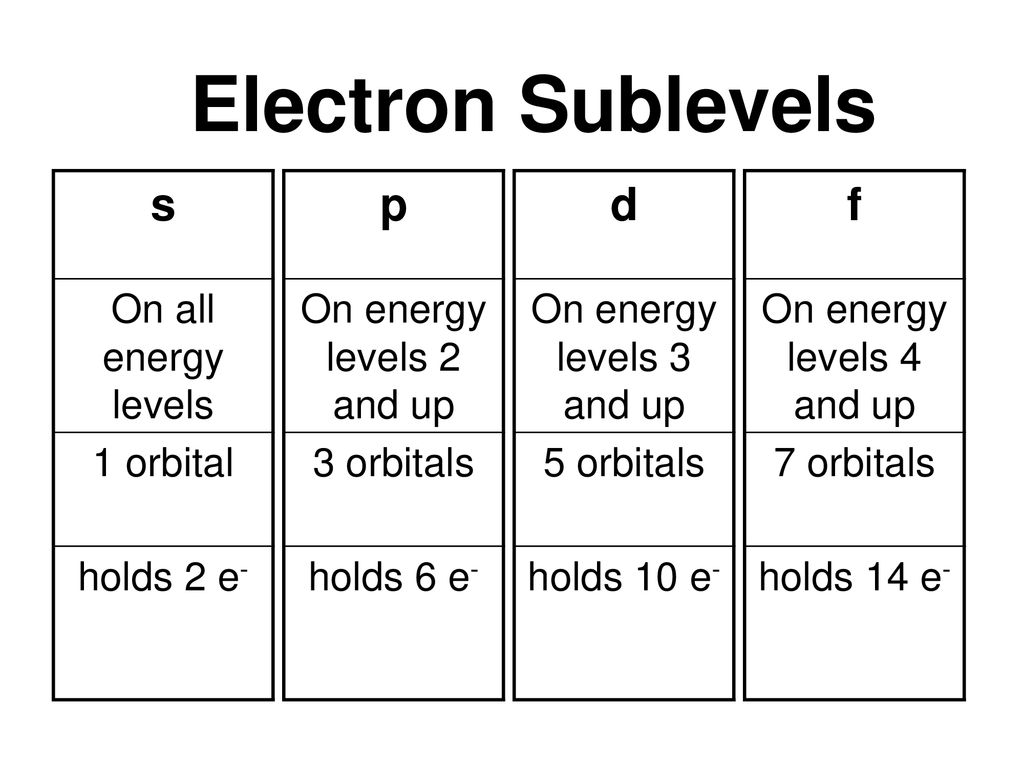
The psublevel is made up of a 3 identical dumbbell like orbitals Each one is situated on its own axis They are at 90 o angles from one and other The dsublevel is made up of a 5 different orbitals and the sublevel holds a maximum of 10 electrons The fsublevel is made up of a 7 different orbitals and holds a maximum of 14 electronsL – Secondary Quantum Number/Orbital Shape Quantum number represents the shape of the orbital s, p, f, d l is a range of n1 a 4 s1, p3, d5, f7= 16 orbitals b 6 9 orbitals 7 Write the complete set of quantum numbers that represent the valence electrons for the following elements aFor example, 3d xy, 3d yz, 3d zx, 3d x 2y 2
Each value of \(l\) indicates a specific s, p, d, f subshell (each unique in shape) The value of \(l\) is dependent on the principal quantum number \(n\) the value of 2l1 will be three and there will be three different orbitals The names of the orbitals are named after the subshells they are found in s orbitals p orbitals d orbitals fOrbitals Chemistry (s, p, d, and f Orbital) Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry Learn more about atomic orbital at Byjus2s is lower energy than 2p)(image source)So for example,
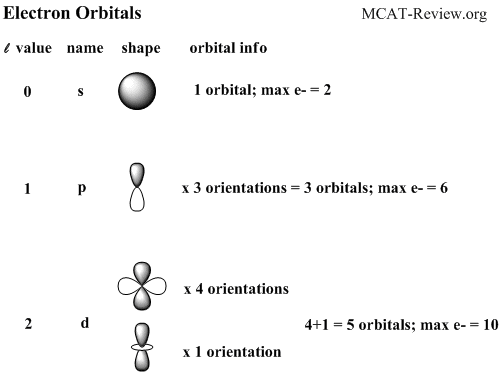
The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively These names, together with the value of n, are used to describe the electron configurations of atomsThe subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum s 1 orbital, 2 electrons p 3 orbitals, 6 electrons d 5 orbitals, 10 electrons f 7 orbitals, 14 electronsThe azimuthal (or orbital angular momentum) quantum number describes the shape of a given orbital It is denoted by the symbol 'l' and its value is equal to the total number of angular nodes in the orbital A value of the azimuthal quantum number can indicate either an s, p, d, or f subshell which vary in shapes This value depends on (and


How Were The Shapes Of S P D And F Orbitals Determined How Did They Get Their Names Of S P D And F Socratic


Parsing The Spdf Electron Orbital Model
Fred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do withThe shapes of the other orbitals are more complicated The letters s, p, d, f, originally were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental, before the relation between spectra and atomic electron configuration was known4 Spin Quantum Number (ms) m s = ½ or ½ Specifies the orientation of the spin axis of an electron An electron can spin in only one of two directions (sometimes called up and down) The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states thatno two electrons in the same atom can have identical values for all four of their quantum numbers



12 1 5 Draw The Shape Of An S Orbital And The Shapes Of The P X P Y And P Z Orbitals Youtube



What Is Spdf Configuration Chemistry Stack Exchange
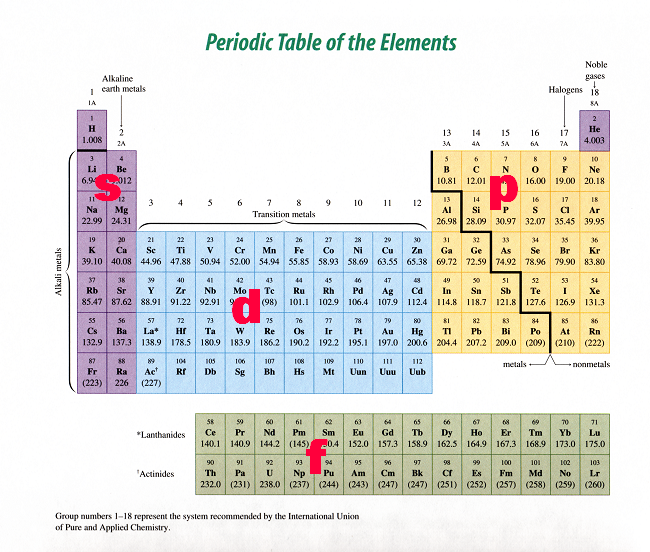
Describe the shapes of s, p, and d orbitals How are these orbitals related to the quantum numbers n, \ell, and m_{\ell} ?Corresponds to the block grouping s,p,d,f on the Periodic Table orbitals regions within electron cloud where electrons orbit the nucleus;The porbitals of higher energy levels have similar shapes although their size are bigger Shape of dorbitals For dsubshell, l = 2, there are five values of m namely 2, 1, 0, 1, 2 It means d orbitals can have five orientations These are represented by d xy, d yz, d zx, d x 2y 2 and d z 2;


S P D F Orbitals Chemistry Socratic


High School Chemistry Shapes Of Atomic Orbitals Wikibooks Open Books For An Open World
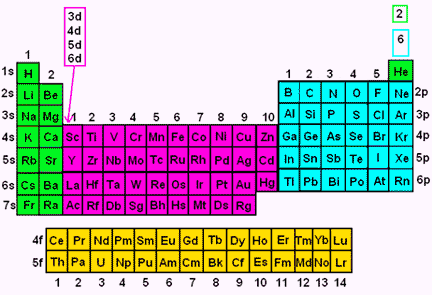
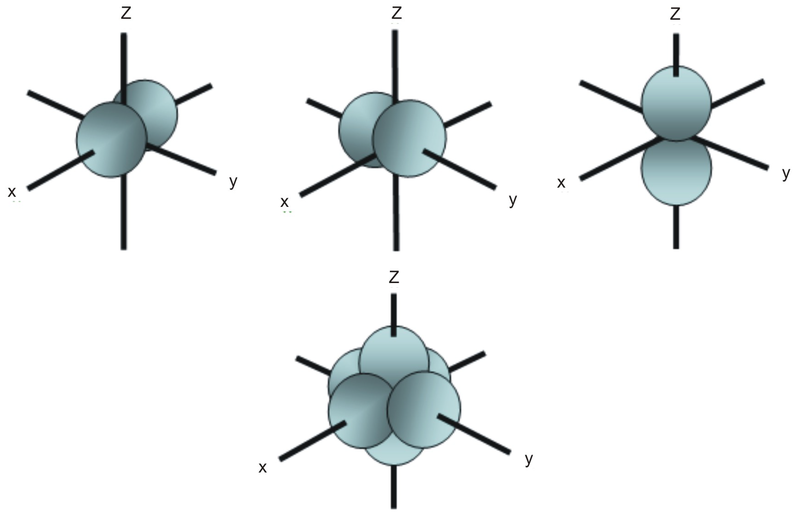
All levels except the first have p orbitals d ORBITALS In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz)S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;Regions within the energy levels;



Shapes Of Atomic Orbitals S P D And F Youtube


S P D F Orbitals Chemistry Socratic
D and f orbitals In addition to s and p orbitals, there are two other sets of orbitals that become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z) At the third level there are nine totalF Orbital The sequence for the f block is unique Beginning with lanthanum (Z=57) it starts a block that contains 15 elements The 5 th level of a tetrahedron has 15 units There are 15 elements for the f block (Z=57 to 71), although an odd number affects the number of orbitals (14 / 2 = 7) It converts a proton to neutron in the next d block to compensate, beginning with the 5d blockD and f orbitals In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z) At the third level there are a total of



Shapes Of Atomic Orbitals Shape Determination Spd Videos Examples
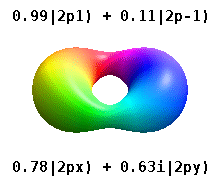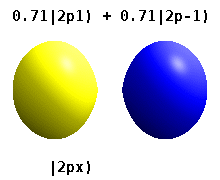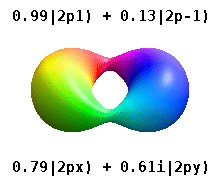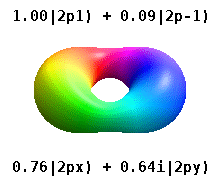


Atomic Orbital Wikipedia
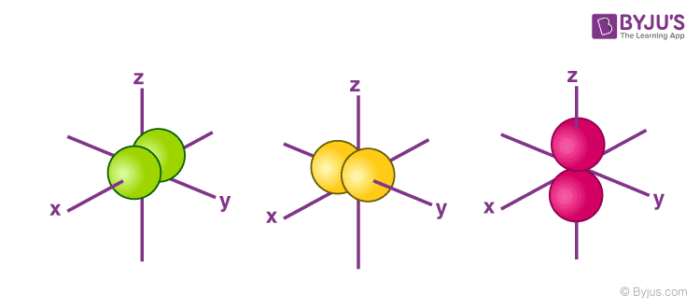
For p orbital Azimuthal quantum number l = 1 and the magnetic quantum number m = 1, 0, 1 Hence p orbitals have three orientations in space Thus p orbital corresponds to dumbbelled shape with the atomic nucleus at its center p orbitals have two lobes directed on opposite sides of the nucleusTHE d ORBITALS In the third energy level, five d orbitals are present They have complicated names and shapes The 3s and 3p (3px, 3py 3px) are present too A total of nine orbitals are found in the third energy level The five 3d orbitals are named;Are to arrange an orbital of that shape around the nucleus "s" subshell One possible orientation "p" subshell Three possible orientations There are five possible orbitals in a "d" subshell, and 7 possible orbitals in an "f" subshell!


Parsing The Spdf Electron Orbital Model


Atomic Orbital Wikipedia
Maximum 6 electrons in 3 orbitals Maximum 2 electrons in 1 orbital Maximum 10 electrons in 5 orbitalsD and f orbitals In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z) At the third level there are a total ofP Orbitals (l=1) Only s orbitals are spherically symmetrical As the value of l increases, the numbe\(r\) of orbitals in a given subshell increases, and the shapes of the orbitals become more complex Because the 2p subshell has l = 1, with three values of m l (−1, 0, and 1), there are three 2p orbitals Figure \(\PageIndex{3}\) Electron Probability Distribution fo\(r\) a Hydrogen 2p
:max_bytes(150000):strip_icc()/antibonding-5b54ef9046e0fb005b6d11a9.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers


How To Draw All 5 D Orbitals Quora
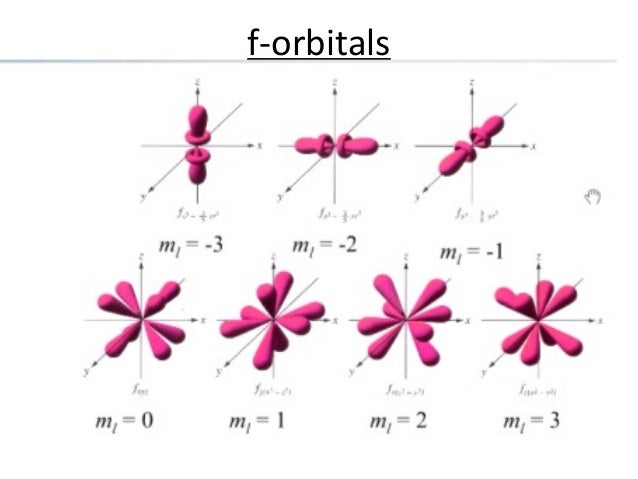
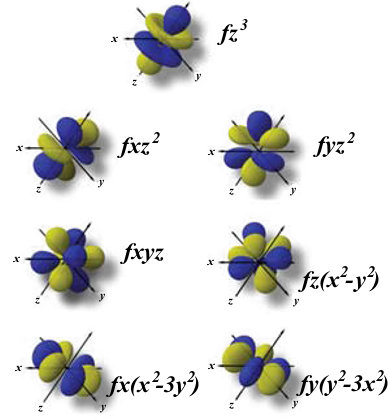
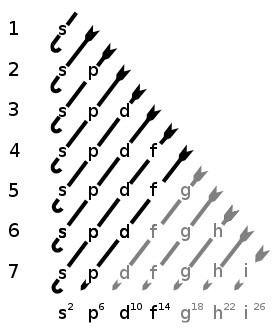
Can hold a maximum of 2 electrons;This video lecture explains shapes and geometry of four different types of Atomic orbitals ie, s, p, d and f orbitalsThe directions, names and the shapes of these orbitals are as follows forbitals The forbitals also have different shapes and these are only available when principal quantum number n = 4 or more When n = 4, l = 3, then m = 3, 2, 1, 0, 1, 2 and 3 That means seven dorbitals are available in an atom The directions, names and the



How To Draw All 5 D Orbitals Quora


Shapes Of Orbitals And Sublevels
Shapes of atomic orbitals The atomic orbitals differ in shape That is, the electrons they describe have different probability distributions around the nucleus Indeed, a part of the reason why orbitals differ in energy is that the electrons that occupy them are likely to be found in different regions around the parent nucleus and hence experience the latter's attraction with differentDescribe the shapes of s, p, and d orbitals How are these orbitals related to the quantum numbers n, \ell, and m_{\ell} ?2s is lower energy than 2p)(image source)So for example,



What Is The Shape Of F Orbital Example


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital
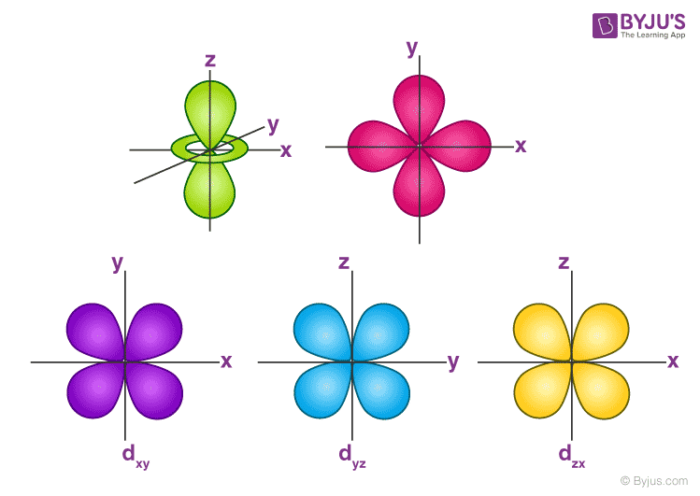
This video explains s, p, d, and f orbitals, sublevels, and their shapes It discusses the 4 quantum numbers n, l, ml, and ms n represents the energy leveThere are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental) Within each shell of an atom there are some combinations of orbitals In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4The values of ml corresponding to d orbital are (–2, –1, 0, 1 and 2) for l = 2 therefore, there are five d orbitals The five dorbitals are assigned with the designation dxy, dyz, dxz, dx 2 –y 2 and dz 2 The energy of all five orbitals are equal but the first four orbitals are similar in shape to each other while the dz 2 differ from



What Are Orbitals And Its Shapes S P D F Orbitals Shapes Class 9 Class 11 Chemistry Youtube Youtube


Chem 101 Week 8 Ch7
Maximum 6 electrons in 3 orbitals Maximum 2 electrons in 1 orbital Maximum 10 electrons in 5 orbitalsAll levels except the first have p orbitals d ORBITALS In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz)Shape of the s, p, d, and f orbitalsChemistry Lecture #26For a pdf transcript of this lecture, go to wwwrichardlouiecom


Spdf Orbitals Can Hold How Many Electrons Qecs Bamagien Site



Origin Of Spdf Orbitals Youtube
S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively These names, together with the value of n, are used to describe the electron configurations of atomsShapes of orbitals An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum The sh



S P D F Obitals Notation Shapes Diagrams How To Work Out Electron Arrangements Configurations Order Of Filling Quantum Levels Electronic Structure Of Atoms Gce A Level Revision Notes


Vixra Org Pdf 1308 0130v1 Pdf
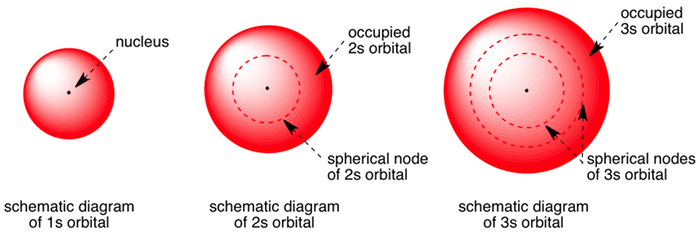
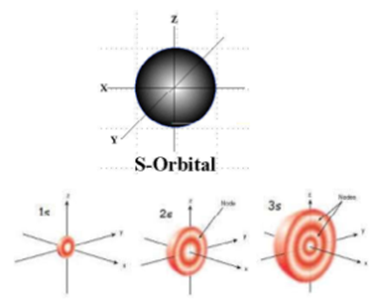
Here you will learn all about your basic ideas, techniques, termiFred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do withS Orbital Versus P Orbital While orbital numbers (eg, n = 1, 2, 3) indicate the energy level of an electron, the letters (s, p, d, f) describe the orbital shape The s orbital is a sphere around the atomic nucleus Within the sphere there are shells in which an electron is more likely to be found at any given time The smallest sphere is 1s


S P D F Orbitals Chemistry Socratic


Atomic Orbitals Definition Shapes Examples And Diagrams
Shapes of sorbitals The sorbitals are spherically symmetrical about the nucleus It implies that, p subshell have three orbitals called as p x, p y and p z Shape of porbitals We have three porbitals, commonly known as p x, p y and p z These three porbitals, possesses equivalent energy and therefore, have same relation with thenucleusThis video explains s, p, d, and f orbitals, sublevels, and their shapes It discusses the 4 quantum numbers n, l, ml, and ms n represents the energy leveAll levels except the first have p orbitals d ORBITALS In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz)
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


S P D F Orbitals And Angular Momentum Quantum Numbers
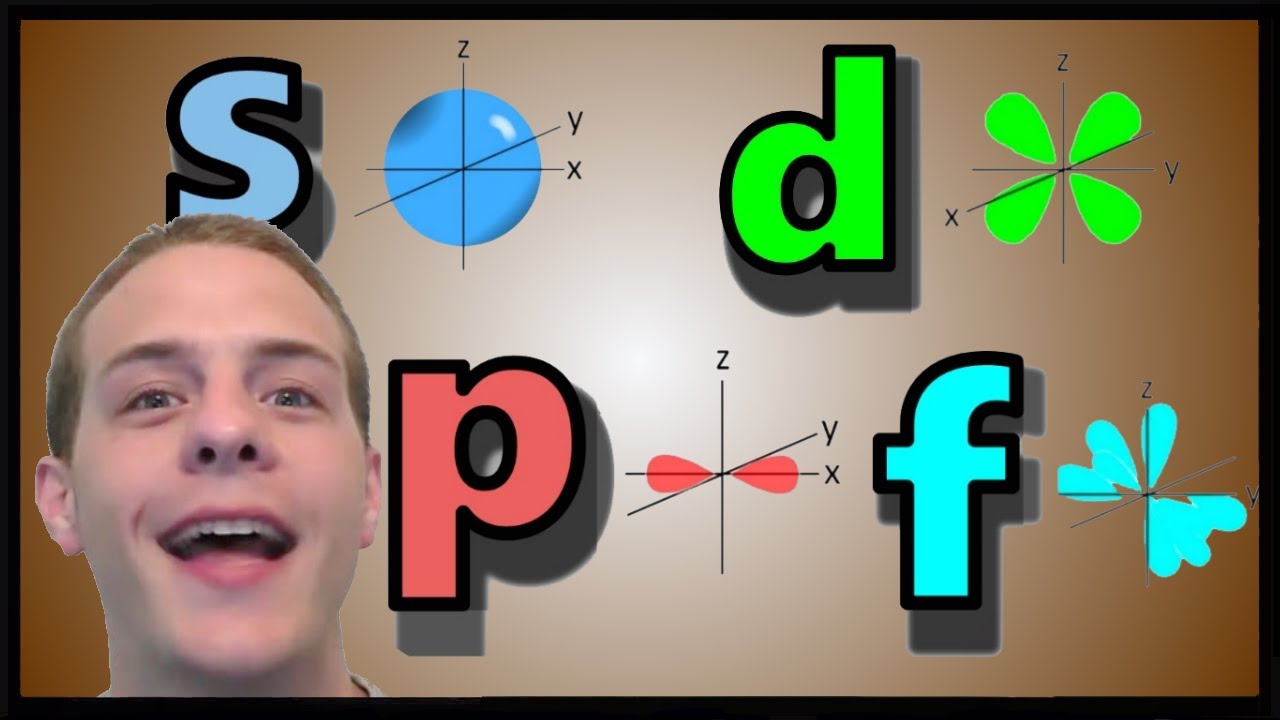


The Shapes Of Atomic Orbitals Youtube
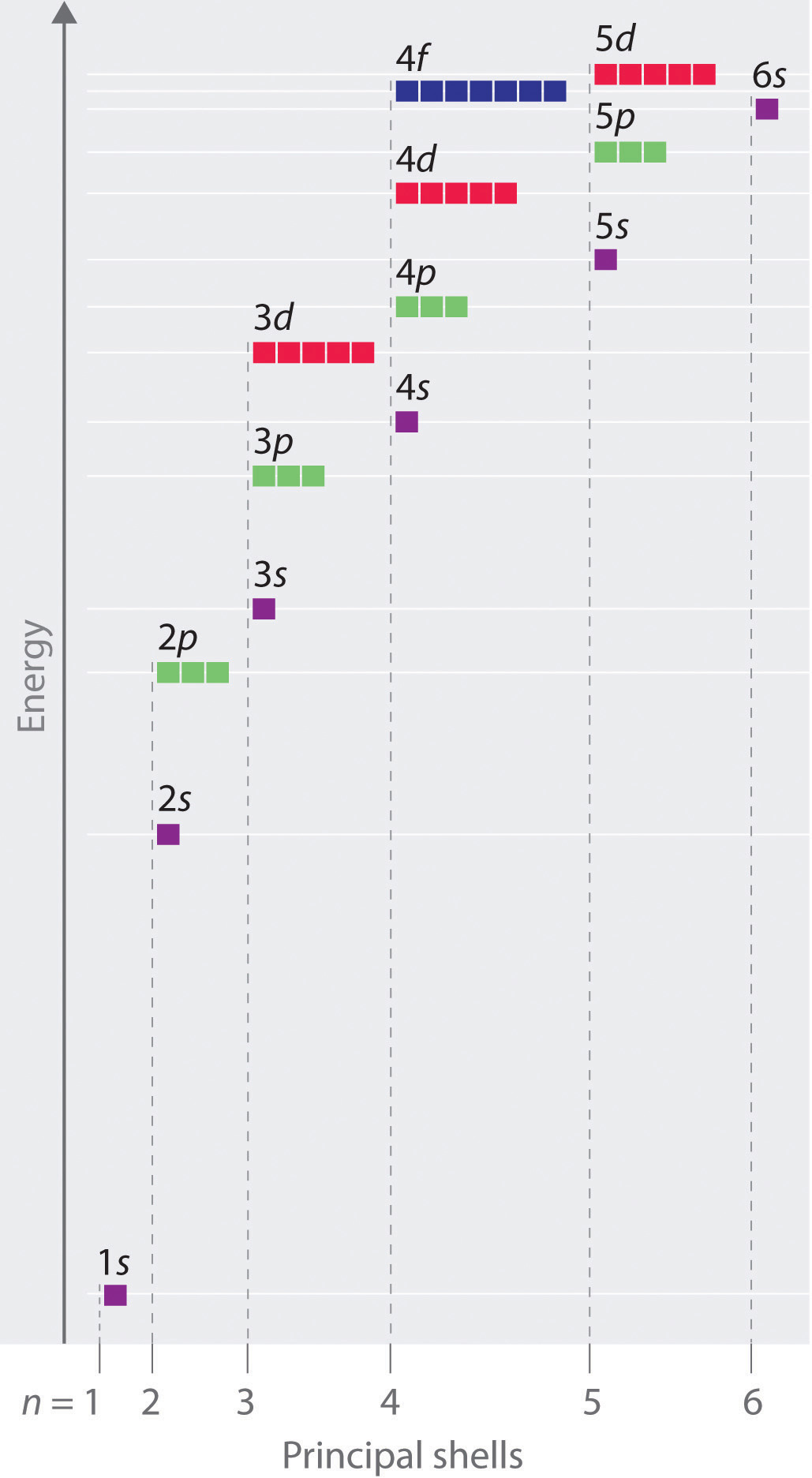
Shapes of Orbitals and Electron Density Patterns The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z) It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily)However, if you look at a crosssection of an orbital, it isn't uniformHow Orbitals are oriented in space?shapes of s, p, d and f orbitals Orbitals In spaceHi!Larger elements have additional orbitals, making up the third electron shell Subshells d and f have more complex shapes and contain five and seven orbitals, respectively Principal shell 3n has s, p, and d subshells and can hold 18 electrons Principal shell 4n has s, p, d, and f orbitals and can hold 32 electrons


Q Tbn And9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau


Electron Configuration Wyzant Resources
Shapes of sorbitals The sorbitals are spherically symmetrical about the nucleus It implies that, p subshell have three orbitals called as p x, p y and p z Shape of porbitals We have three porbitals, commonly known as p x, p y and p z These three porbitals, possesses equivalent energy and therefore, have same relation with thenucleusYou will see the lowercase letters s, p, d, f, g, and h for the suborbitals For example, the electron in a hydrogen (H) atom would have the values n=1 and l=0 The single electron would be found in the "K" shell and the "s" suborbital If you go on to learn more about chemistry, you may see its description written as 1s1Review Question List the four orbital shapes The orbital shapes are s, p, d, and f Summarize Aufbau's rule for filling orbitals Electrons fill orbitals with the
:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)


S P D F Orbitals And Angular Momentum Quantum Numbers


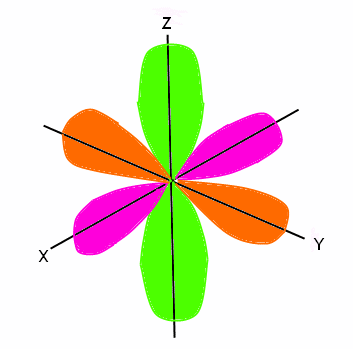
Shapes Of Atomic Orbital Chemistry Class 11 Structure Of Atom
Review Question List the four orbital shapes The orbital shapes are s, p, d, and f Summarize Aufbau's rule for filling orbitals Electrons fill orbitals with theAn illustration of the shape of the 3d orbitals Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values m l =0, ±1, ±2,Footnotes (1) Each subshell is made up of a set of orbitals, the orbitals reflect which subshell they belong to by using the same letter, that is, there are s orbitals, p orbitals, d orbitals and f orbitals However, although there is only one s orbital in the s subshell, there are 3 p orbitals in the p subshell, 5 d orbitals in the d subshell, and 7 f orbitals in the 5 subshell



Draw The Shapes Of S P And D Orbitals Brainly In


S Subshell Shape Photos Download Jpg Png Gif Raw Tiff Psd Pdf And Watch Online
Number of orbitals differs with sublevel type (s,p,d,f)


Parsing The Spdf Electron Orbital Model


Shapes Of Atomic Orbital Chemistry Class 11 Structure Of Atom


Q Is It Possible For An Atomic Orbital To Exist Beyond The S P F And D Orbitals They Taught About In School Like Could There Be A Other Letter Orbital Beyond



Shape Of The P1 2 Orbital Chemistry Stack Exchange



Sublevel Spdf Chart Fgkb Katasekan Site


Q Tbn And9gcskzrg40udovpzcheo8bco5bn Kwmhapjcvntj Svtxzvm B8yr Usqp Cau



Why Different Orbitals Have Different Shapes Britannica


Atomic Orbitals


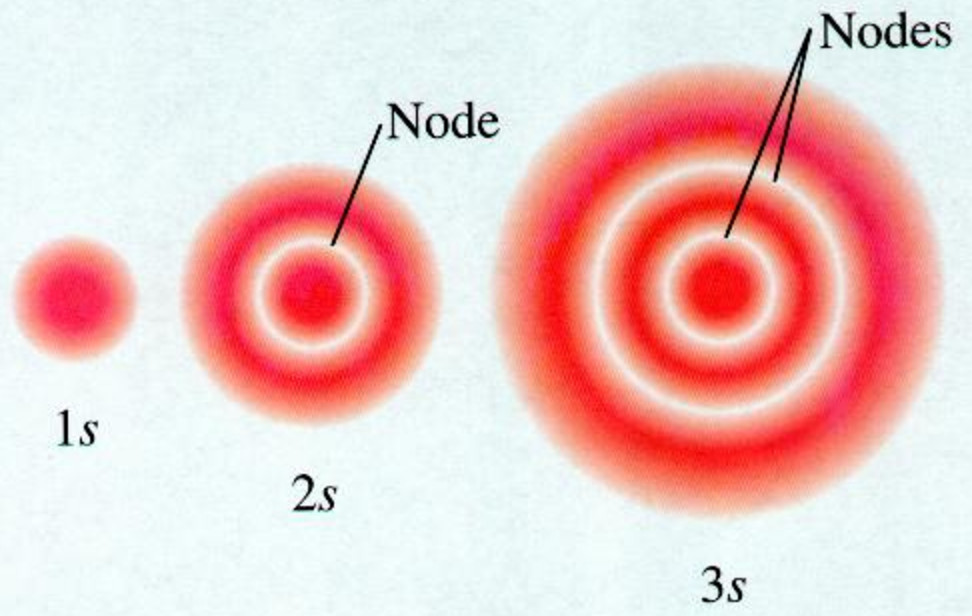
Shape Of S Orbitals In 3d


Electron Configuration Wyzant Resources



S P D F Orbitals Chemistry Socratic


What Are The Orbital Shapes Of S P D And F Socratic
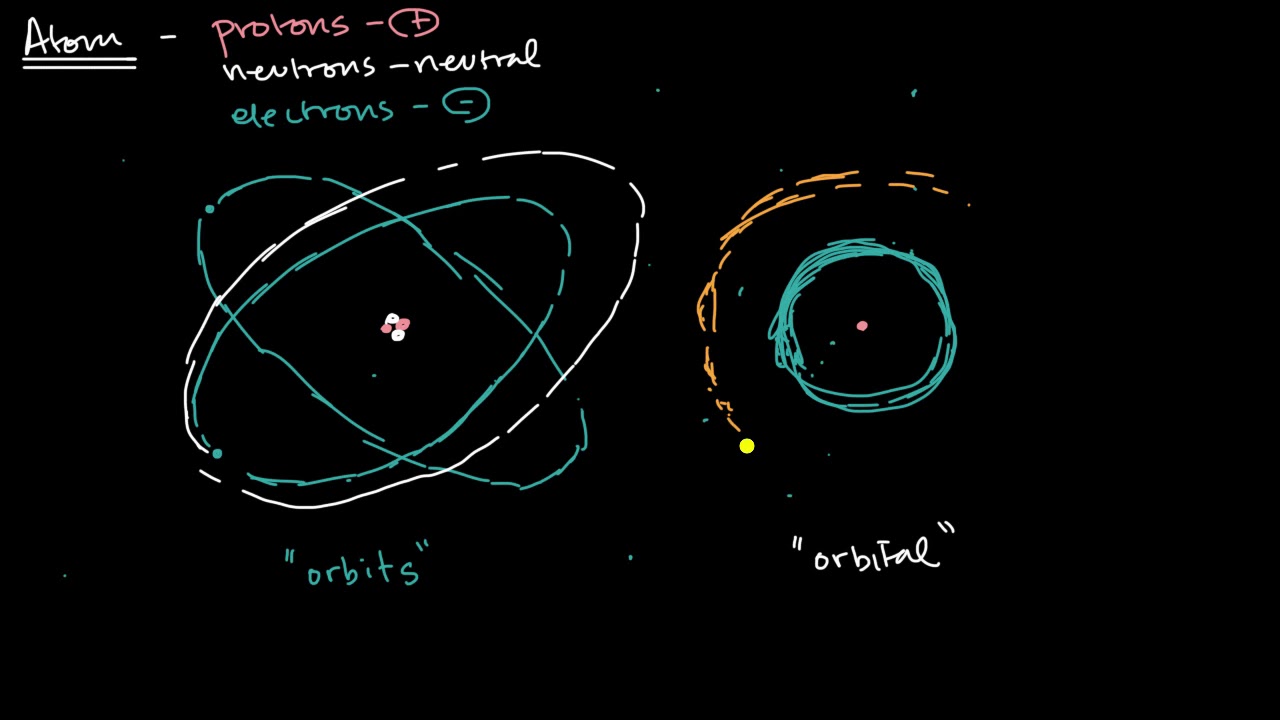


Shells Subshells And Orbitals Video Khan Academy


S P D F Orbitals Meaning Mypawl
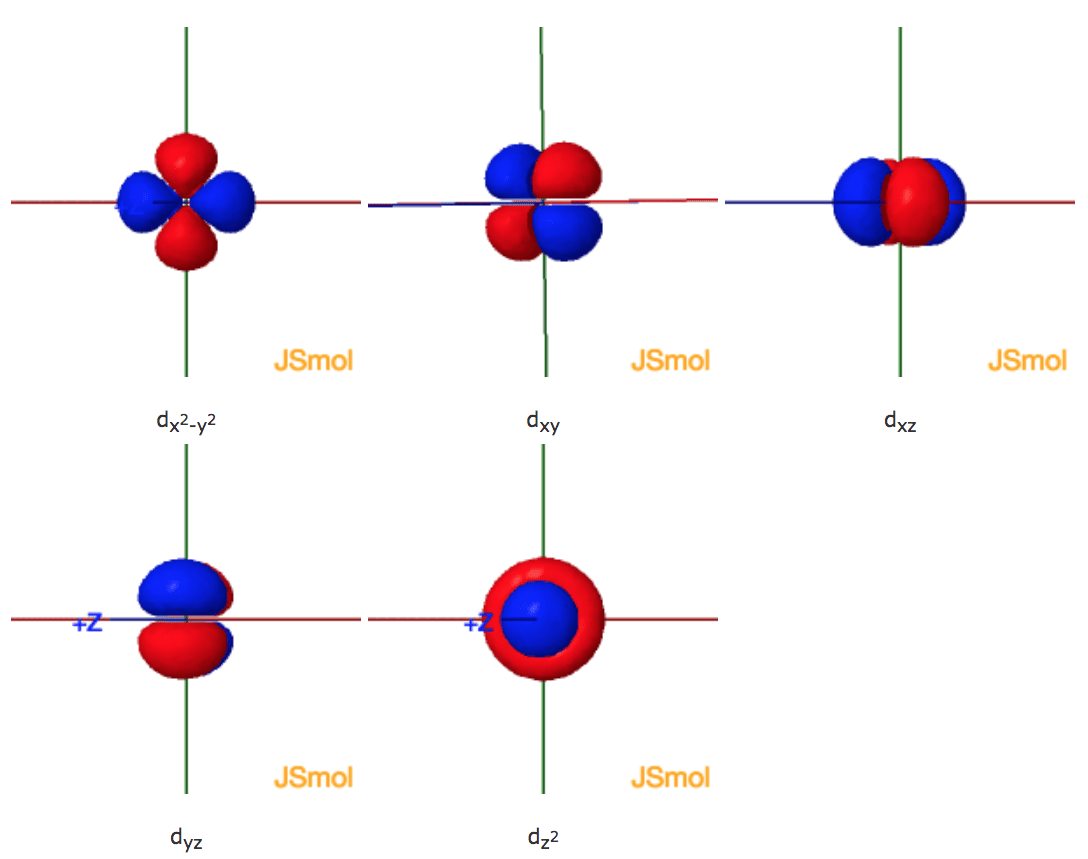


Shapes Of The 3d Orbitals In 3d
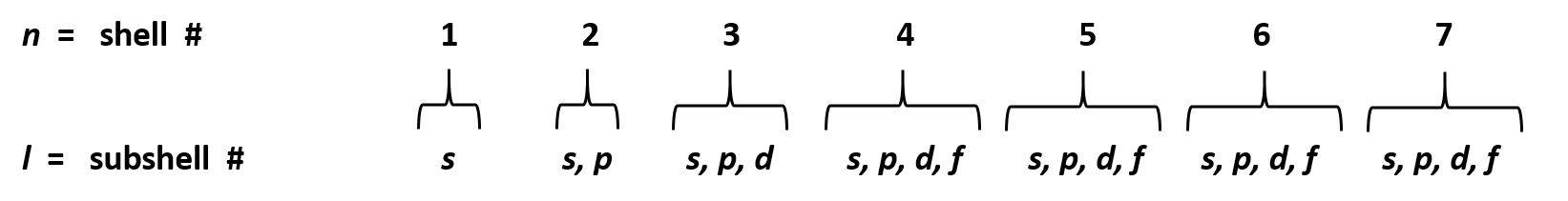


Ch150 Chapter 2 Atoms And Periodic Table Chemistry
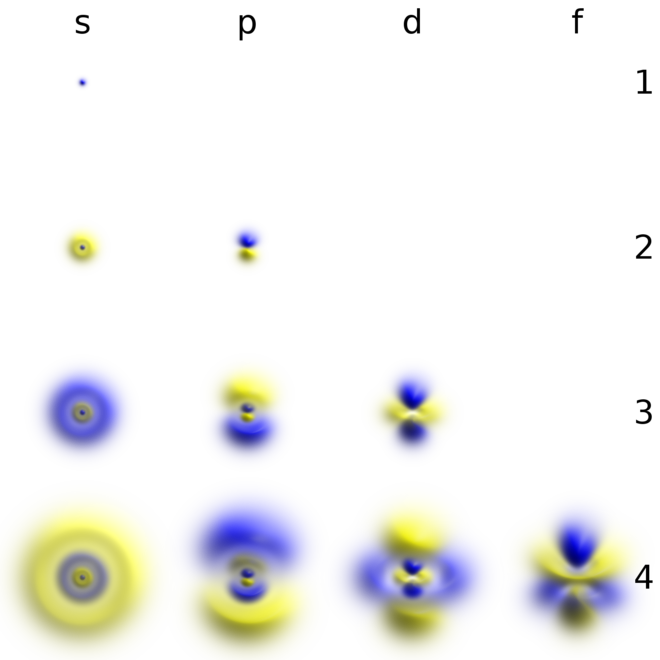


Atomic Orbital Wikiwand



Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital


Q Tbn And9gcqulzhcplpqcomqengxsfh8zlrg Jw3 8b 3am6v0f U Tzuel Usqp Cau


What Is The Structure Of An F Orbital Quora


Four Types Of Orbitals Their Shapes
/atomic-nucleus-and-orbiting-electrons-475158093-58ebfd365f9b58ef7ead201c.jpg)


Orbital Definition And Example



Draw The Shape Of S And P Orbitals Brainly In



Quantum Number Wikipedia


Quantum Mechanic Model


Parsing The Spdf Electron Orbital Model



Electron Orbital Definition Shells Shapes Video Lesson Transcript Study Com



6 6 The Shapes Of Atomic Orbitals Chemistry Libretexts


An Atomic Model Our Present Model Of The Atom Is Based On The Concept Of Energy Levels For Electrons Within An Atom And On The Mathematical Interpretation Of Detailed Atomic Spectra The Requirements For Our Model Are Each Electron In A Particular Atom



Shape Of Orbitals
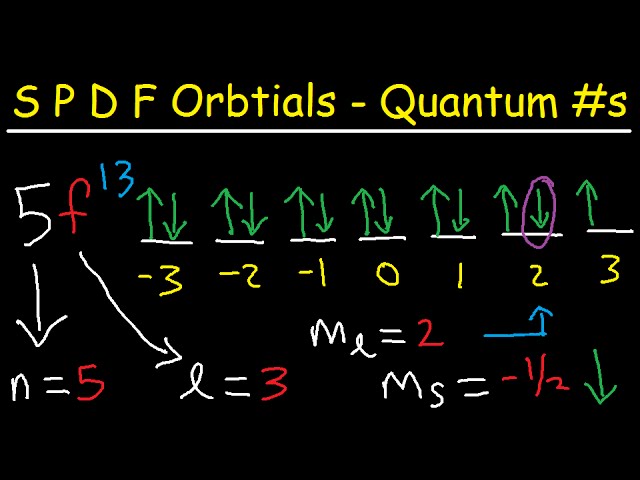


S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube



Shapes Of S And P Orbitals As Chemistry Youtube


Orbitals And Their Types S P D F Orbitals And Their Shapes


Shapes Of Orbitals And Sublevels



Chemistry 103 Lecture Ppt Video Online Download


Q Tbn And9gcqgp28lgnr6xcufpgiroedaenthgrys Oedjaqmrwowa3gfhdra Usqp Cau



Atomic Orbital Wikipedia


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital



Atomic Orbital Wikipedia



S P D F Orbitals And Angular Momentum Quantum Numbers


How Were The Shapes Of S P D And F Orbitals Determined How Did They Get Their Names Of S P D And F Socratic



900 Fioso Nano Tech Pins Ideas Physics Research Material Science Physics


Parsing The Spdf Electron Orbital Model
:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers
:max_bytes(150000):strip_icc()/ShellAtomicModel-5a6ab592aded4bb7a1328f809e4f10da.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers
:max_bytes(150000):strip_icc()/GettyImages-1157225833-f294a1f0fa314b12bf2da395e95107d7.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers


Q Is It Possible For An Atomic Orbital To Exist Beyond The S P F And D Orbitals They Taught About In School Like Could There Be A Other Letter Orbital Beyond


S P D F Orbitals Chemistry Socratic


Parsing The Spdf Electron Orbital Model


How Do You Draw S P D F Orbitals Socratic


S P D F Orbitals Chemistry Socratic



Angular Momentum Quantum Number Definition Example Video Lesson Transcript Study Com



Orbitals The Basics Atomic Orbital Tutorial Probability Shapes Energy Crash Chemistry Academy Youtube



High School Chemistry Shapes Of Atomic Orbitals Wikibooks Open Books For An Open World
:max_bytes(150000):strip_icc()/GettyImages-1131590633-8dac52a0551c415a81278874de72b3ff.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers


Chem4kids Com Atoms Orbitals


Ch 9



High School Chemistry Shapes Of Atomic Orbitals Wikibooks Open Books For An Open World


Shapes Of Orbitals And Sublevels



Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital


Chapter 2 5 Atomic Orbitals And Their Energies Chemistry Libretexts


Parsing The Spdf Electron Orbital Model



What Is Spdf Configuration Chemistry Stack Exchange



The Actinide Research Quarterly 1st Quarter 04


How Were The Shapes Of S P D And F Orbitals Determined How Did They Get Their Names Of S P D And F Socratic


Electron Configuration Chemistry 10
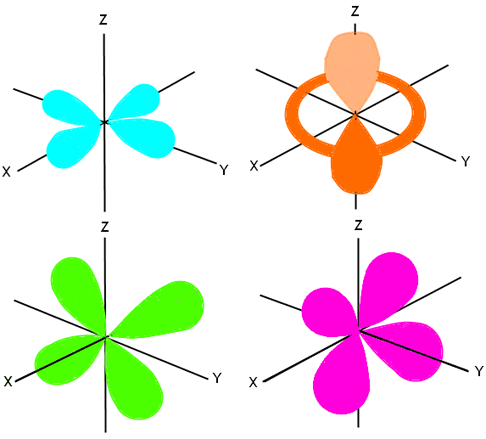


Electron Configuration Wyzant Resources



What Is The Shape Of An F Orbital Quora


Shapes Of Orbitals S P D Shapes



You Are At Miracle Mbalisi S Blog Now Feel Free Understanding Atomic Orbitals Spdf And Their Shape


コメント
コメントを投稿